How Can Substances in a Mixture Be Separated
Separating Mixtures
1 of the most important things that chemists practice is separate mixtures. For example, when I was working in a chemistry lab and I would go out for dejeuner, information technology was of vital interest to me that I effectively separate the pickles from the residuum of the hamburger, lest I become sick later in the mean solar day. Other chemists I know have also found the power to separate mixtures to exist of import when eating trail mix or scraping mud from their shoes. More often than not, I've found it's easier to carve up the components in a heterogeneous mixture (e.g., the pickles from hamburgers) than the components in a homogeneous mixture (due east.yard., the rum from a Pia Colada) because information technology'due south easier to pick things apart when you can see the dissimilar components.
Information technology'due south good that chemists become so much practice separating mixtures similar these in their everyday lives considering mixture separation is important for other purposes also. Let's accept a look.
Filtration
1 of the simplest methods used to split mixtures is filtration. If 1 of the components is a liquid and the other is a solid, filtration is as easy as pouring the whole mixture through filter paper. An everyday instance of filtration can be seen in a coffee maker, where the coffee passes through a paper filter but the grounds do not.
Distillation
When one chemical compound is dissolved in another, or when two liquids are mixed together, the most commonly used method to carve up them is distillation. In a distillation, the mixture is slowly heated over a Bunsen burner or hotplate. Because the components in a mixture accept different boiling points, ane of them will eddy before the other. The vapor from this compound can be nerveless from a condenser, enabling information technology to exist isolated in a pure form. A distillation apparatus is shown in the following figure.
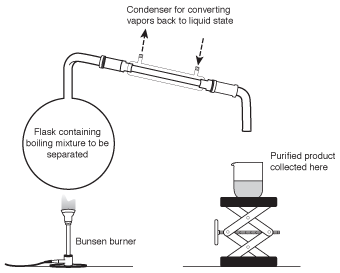
Figure 6.3A distillation appliance (oftentimes referred to as a "still") uses the different humid points of the components in a mixture to separate them.
Chromatography
At some time or another, all of the states take marked our shirt with a pen. Sometimes we go lucky and the ink doesn't stick well to the material?in these cases, nosotros can clean the shirt by putting it in the wash. Sometimes we get very unlucky and the ink sticks to the fabric and so well that it'southward there for good, no matter how many times it'due south washed with bleach and detergent.
In the same way, chemic substances can frequently be separated from one some other based on how well they stick to a solid. The apply of this difference in "stickiness" to divide the components of a mixture is referred to as chromatography.
Typically, chromatography is performed by placing a mixture of two or more than chemicals into a drinking glass column filled with silica. When an organic solvent such as ethyl acetate or alcohol is poured through the cavalcade, ane of the components of the mixture will tend to stick to the silica better than the other. As a result, the less sticky i volition laissez passer through the column more quickly, while the stickier 1 will accept a little longer.
Extraction
Let's say that yous have a chemical compound dissolved in a liquid that y'all desire to remove. For instance, you accept a pocket-sized amount of salt dissolved in oil and desire to remove information technology. How would you do this?
Though distillation could practise the task, it takes a long fourth dimension and considerable endeavour. An alternating mode of making this separation is to detect a liquid that isn't soluble with the first liquid and that's meliorate at dissolving the salt than the oil is. When the two liquids are mixed and shaken, the table salt will tend to motion from the oil (where it'southward not very soluble) into the h2o (where it is). When this process is consummate, it's a simple matter to pour out the water, leaving behind the pure oil.
Excerpted from The Consummate Idiot's Guide to Chemistry 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in office in any form. Used by arrangement with Blastoff Books, a member of Penguin Group (USA) Inc.
To club this book directly from the publisher, visit the Penguin U.s. website or phone call i-800-253-6476. You tin can also purchase this volume at Amazon.com and Barnes & Noble.
- Chemical science: Shake It Up: Mixtures
Source: https://www.infoplease.com/math-science/chemistry/chemistry-separating-mixtures
Belum ada Komentar untuk "How Can Substances in a Mixture Be Separated"
Posting Komentar